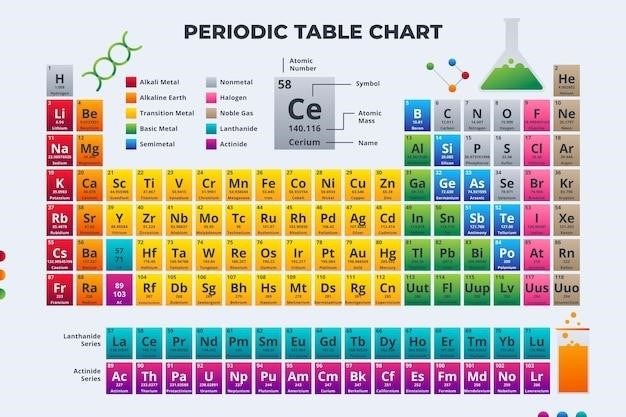
The Periodic Table⁚ A Webquest Adventure
Embark on a captivating journey through the world of chemistry with this interactive webquest. Explore the fascinating periodic table, unravel its secrets, and test your knowledge with engaging activities. Get ready to delve into the building blocks of matter and discover the intriguing relationships between elements. This webquest provides a comprehensive learning experience that will deepen your understanding of the periodic table and its importance in the realm of science.
Introduction to the Periodic Table
The periodic table is a fundamental tool in chemistry, organizing all known chemical elements in a systematic manner. It’s like a map of the building blocks of matter, revealing their properties and relationships. Each element occupies a specific position on the table based on its atomic number, which represents the number of protons in its nucleus; The table is arranged into rows called periods and columns called groups. Elements within the same group share similar chemical properties due to having the same number of valence electrons, the electrons in their outermost shell.
The periodic table is not just a static chart; it’s a dynamic representation of the periodic law, which states that the properties of elements are periodic functions of their atomic numbers. This means that as you move across or down the table, certain properties like electronegativity, ionization energy, and atomic radius exhibit predictable trends. Understanding these trends is crucial for predicting the behavior of elements and their reactions.
The periodic table is a powerful tool for scientists, allowing them to understand the behavior of elements and predict their reactions. It is a cornerstone of chemistry, providing a framework for understanding the vast diversity of matter in the universe.
Exploring the Periodic Table
The periodic table is a treasure trove of information about the elements. To navigate this vast landscape, you need to understand its structure and how elements are categorized; The table is divided into periods, which are horizontal rows, and groups, which are vertical columns. Each element has a unique symbol, atomic number, and atomic mass. The atomic number represents the number of protons in an atom’s nucleus, which defines the element. The atomic mass is the average mass of an atom of that element, taking into account its isotopes.
The periodic table also features various blocks that classify elements based on their electron configurations. The s-block elements are found in groups 1 and 2, the p-block elements in groups 13 to 18, the d-block elements in groups 3 to 12, and the f-block elements in the lanthanide and actinide series. These blocks provide insights into the elements’ chemical behavior and bonding properties.
As you explore the periodic table, you’ll notice certain trends in the elements’ properties. For instance, elements within the same group share similar chemical characteristics due to having the same number of valence electrons. Understanding these trends is essential for predicting how elements will react with each other and for comprehending the periodic law, which governs the recurring patterns in elemental properties.
Understanding Periodic Trends
The periodic table is not just a random arrangement of elements; it showcases fascinating trends in their properties. These trends are directly related to the arrangement of electrons in an atom’s shells. As you move across a period from left to right, the atomic radius generally decreases, due to an increase in the number of protons in the nucleus, which attracts electrons more strongly. Conversely, moving down a group increases the atomic radius as additional electron shells are added, pushing the outermost electrons further away from the nucleus.
Another significant trend is electronegativity, which measures an atom’s ability to attract electrons in a bond. Electronegativity increases across a period as the effective nuclear charge increases, making the atom more attractive to electrons. However, it decreases down a group as the outermost electrons are further away from the nucleus and less tightly held. Similarly, ionization energy, the energy required to remove an electron from an atom, also increases across a period and decreases down a group, reflecting the increasing attraction of the nucleus for electrons.
Understanding these periodic trends is crucial for predicting chemical behavior and understanding how elements interact. For example, elements with high electronegativity tend to gain electrons and form anions, while those with low electronegativity tend to lose electrons and form cations. These trends are fundamental to understanding the formation of chemical bonds and the diversity of chemical compounds.
Webquest Activities
Prepare to dive into a series of engaging activities designed to enhance your understanding of the periodic table. These activities will take you on a journey through various online resources, allowing you to explore the table’s structure, properties, and historical development. You’ll learn about the fascinating stories behind the discovery of elements, the connections between their properties and their placement on the table, and the practical applications of these elements in our daily lives.
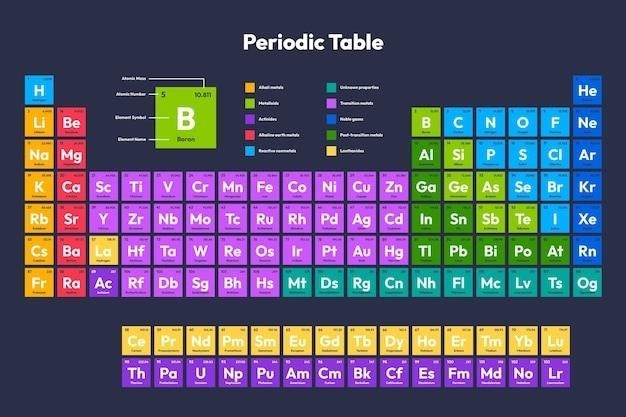
From interactive simulations to detailed online articles, these activities will challenge your knowledge and ignite your curiosity. You’ll have the opportunity to test your understanding of periodic trends, identify elements based on their properties, and discover the importance of the periodic table in various fields, including chemistry, physics, and materials science. Get ready to unravel the mysteries of the periodic table and expand your scientific horizons.
Remember to document your findings and complete the accompanying worksheet to solidify your understanding. This webquest provides a dynamic and interactive learning experience that will empower you to navigate the periodic table with confidence and apply your knowledge to real-world scenarios.
Element Groups and Properties
The periodic table is a masterpiece of organization, grouping elements with similar properties into vertical columns called groups. Each group represents a family of elements that share a common set of characteristics, often reflecting the number of valence electrons they possess. These valence electrons play a crucial role in determining an element’s reactivity and the types of chemical bonds it can form.
For instance, Group 1, also known as the alkali metals, are highly reactive metals that readily lose one electron to form positive ions. In contrast, Group 18, the noble gases, are inert elements that are reluctant to participate in chemical reactions due to their stable electron configurations. The periodic table provides a framework for understanding the relationships between elements and their behavior, allowing us to predict their properties and reactions based on their group affiliation.
Exploring the properties of different element groups can reveal fascinating patterns and insights into the nature of matter. From the metallic luster of transition metals to the non-metallic nature of halogens, each group exhibits distinct characteristics that reflect their unique electronic configurations. The periodic table serves as a valuable tool for comprehending the diversity and interconnectedness of elements, revealing the underlying principles that govern their behavior.
Interactive Periodic Table Resources
The digital age has revolutionized the way we learn and interact with the periodic table. Numerous online resources offer interactive experiences that make exploring the elements engaging and insightful. These resources often go beyond the traditional static table, providing dynamic visualizations, animations, and interactive features that enhance our understanding of the periodic trends and properties of elements.
For example, some websites allow users to manipulate the periodic table, zooming in on individual elements to reveal detailed information about their atomic structure, electronic configurations, and physical and chemical properties. Others provide interactive quizzes and games that test our knowledge and reinforce key concepts. These digital tools offer a powerful means of engaging with the periodic table, fostering a deeper understanding of its structure and the relationships between elements.
By leveraging these interactive resources, we can embark on a dynamic exploration of the periodic table, uncovering hidden patterns and gaining a more comprehensive grasp of the fundamental principles that govern the behavior of matter. These online platforms serve as valuable companions for students, educators, and anyone seeking to deepen their knowledge of the periodic table and its significance in the realm of chemistry.
The History of the Periodic Table
The periodic table, a cornerstone of modern chemistry, has a rich and fascinating history. Its development was a gradual process, with contributions from numerous scientists over several decades. The story begins with the efforts of early chemists to classify elements based on their properties, leading to the identification of groups of elements with similar behaviors.
One of the key figures in this endeavor was Dmitri Mendeleev, a Russian chemist who in 1869 published his version of the periodic table, arranging elements in order of increasing atomic weight. Mendeleev’s table not only organized known elements but also predicted the existence of undiscovered elements, which were later confirmed. His work laid the foundation for the modern periodic table, which continues to evolve as new elements are discovered and our understanding of atomic structure deepens.
The development of the periodic table was a remarkable achievement, demonstrating the power of scientific inquiry and the interconnectedness of different branches of science. It serves as a testament to the ingenuity of scientists who sought to unravel the mysteries of the universe and understand the fundamental building blocks of matter.
Periodic Table Webquest Answer Key
This webquest answer key provides the solutions to the activities and questions posed in the “Periodic Table⁚ A Webquest Adventure” module; It’s designed to help you check your understanding and reinforce your learning about the periodic table, its history, and its applications in the world of chemistry.
For each activity or question, you’ll find the correct answers and explanations, along with relevant information to deepen your knowledge. Use this answer key as a valuable resource to compare your responses, clarify any uncertainties, and expand your understanding of the periodic table.
Whether you’re a student embarking on your first journey through the periodic table or a seasoned learner looking for a refresher, this answer key can serve as a valuable companion. Remember that understanding the periodic table is essential for comprehending the diverse world of chemical elements and their interactions, which are fundamental to our understanding of the universe.
Importance of the Periodic Table
The periodic table, a seemingly simple chart of elements, holds the key to understanding the vast complexity of the universe. It’s a testament to the interconnectedness of all matter, revealing patterns and trends that govern the behavior of elements. Its significance extends beyond the realm of chemistry, impacting fields such as medicine, technology, and environmental science.
From the development of life-saving drugs to the creation of innovative materials, the periodic table serves as a foundational tool for scientific progress. Its ability to predict and explain the properties of elements empowers us to harness their potential for the benefit of humanity.
As you continue your exploration of chemistry, remember that the periodic table is not just a static chart; it’s a dynamic framework that guides our understanding of the world around us. Its importance lies in its ability to connect seemingly disparate concepts and pave the way for new discoveries that shape our future.
References
This webquest has been designed to provide a comprehensive and engaging exploration of the periodic table. The following resources have been used in its development and are recommended for further learning⁚
- RSC Education⁚ Mendeleev’s Periodic System⁚ This website provides interesting facts about the invention of the periodic table and offers valuable insights into the history of this crucial scientific tool. (http://www.rsc.org/education/teachers/lear…p/mendeleev.htm)
- Interactive Periodic Table⁚ This interactive periodic table, created with React.js and CSS Grid, offers a visually appealing and engaging way to learn about the elements. (https://www.youtube.com/watch?v=dQw4w9WgXcQ)
- Periodic Trends Worksheet Answer Key⁚ This worksheet provides a comprehensive overview of periodic trends and helps students understand the relationships between elements. (https://www.youtube.com/watch?v=dQw4w9WgXcQ)
- Periodic Trends Total Review⁚ This resource offers a thorough review of periodic trends, covering key concepts and providing valuable practice exercises. (https://www.youtube.com/watch?v=dQw4w9WgXcQ)
These resources, along with other credible online sources and textbooks, will help you deepen your understanding of the periodic table and its importance in the world of science.